Bethanechol Clinical Trials: Understanding and Interpreting the Evidence
 Oct, 15 2025
Oct, 15 2025
Bethanechol NNT Calculator
Number Needed to Treat Calculator
The Number Needed to Treat (NNT) is a crucial metric for understanding clinical trial results. It represents how many patients need to be treated with Bethanechol to achieve one additional favorable outcome.
When you stumble upon a study about Bethanechol is a synthetic muscarinic agonist that stimulates smooth muscle activity in the bladder and gastrointestinal tract, the numbers and jargon can feel overwhelming. This guide breaks down what the drug does, how its trials are built, which results really matter, and where to spot red flags. By the end you’ll be able to read a Bethanechol paper and walk away with a clear take‑away.
What Bethanechol Is and How It Works
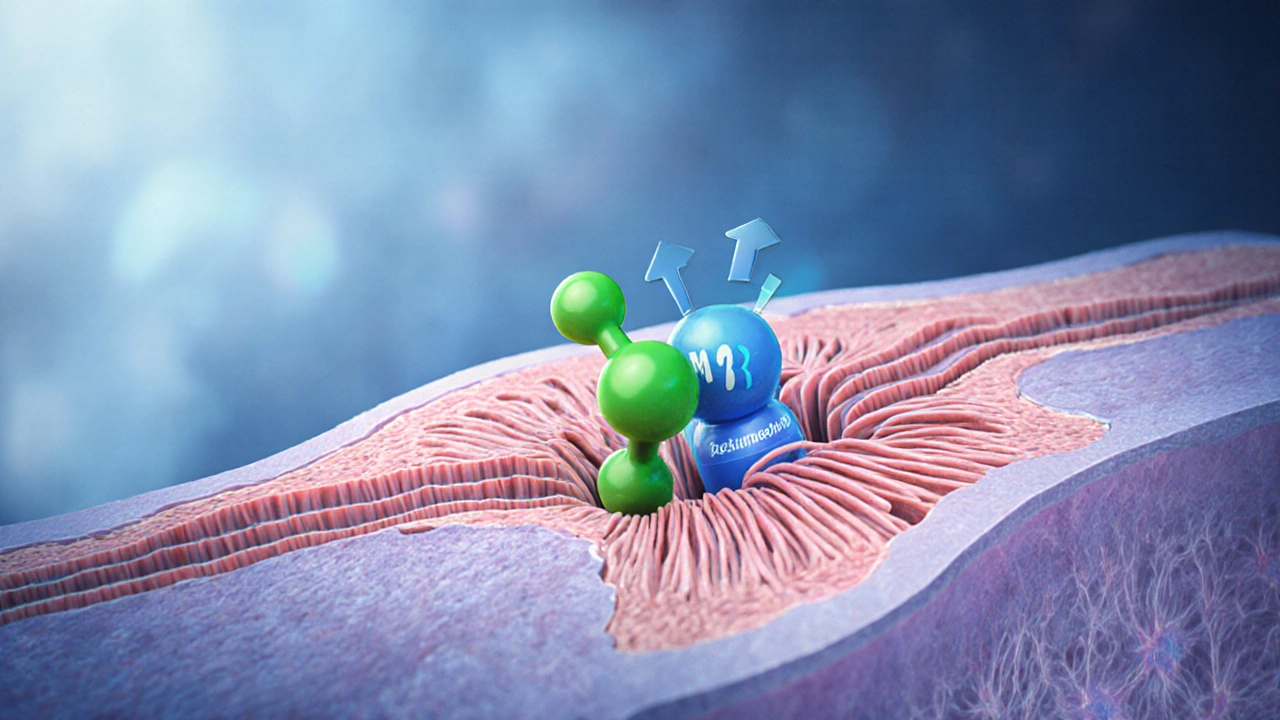
At its core, Bethanechol belongs to the class of muscarinic agonists that bind to M3 receptors on smooth muscle cells, causing contraction without affecting the central nervous system. This makes it useful for two main conditions:
- urinary retention - the drug helps the bladder contract, easing the need for catheterization.
- gastrointestinal motility disorders - it can stimulate peristalsis in patients with postoperative ileus.
Because it acts locally, the safety profile is generally predictable, but dose‑related side effects like sweating, abdominal cramps, and hypotension still pop up in trials.
Designing a Bethanechol Trial: The Basics
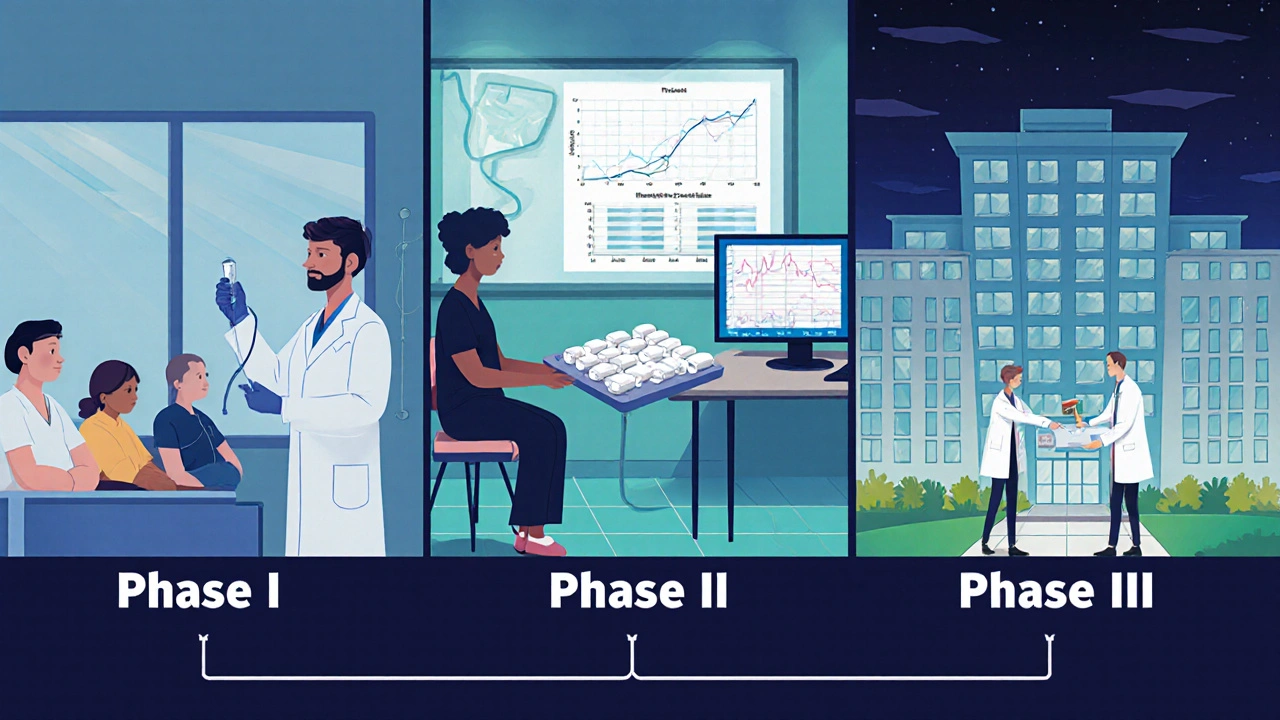
Most modern Bethanechol studies follow the same roadmap as any other drug trial, but a few nuances are worth noting.
- Phase I - Safety First: Small groups of healthy volunteers receive escalating doses to map tolerability and pharmacokinetics.
- Phase II - Proof of Concept: Researchers enroll patients with the target condition (e.g., postoperative urinary retention) and look for a signal of efficacy while continuing safety monitoring. This is where the primary endpoint - often time to first spontaneous void or time to first bowel movement - is chosen.
- Phase III - Confirmatory: Large, multicenter, placebo‑controlled studies compare Bethanechol against standard care or inert pills. Randomization, double‑blinding, and intention‑to‑treat analysis are the hallmarks.
Regulatory bodies such as the FDA scrutinize these phases for both statistical significance (p‑value < 0.05) and clinical relevance (e.g., reduction of catheter‑time by >2hours).
Key Metrics to Watch in Bethanechol Papers
When you open a trial report, focus on the following data points:
- Efficacy outcomes: Mean time to first void, proportion of patients achieving successful voiding within 6hours, or gastrointestinal recovery scores.
- Safety outcomes: Incidence of adverse events such as bradycardia, excessive salivation, or abdominal pain.
- Confidence intervals: Look for 95% CI around the effect size; narrow intervals signal precise estimates.
- Number needed to treat (NNT): An NNT of 4 for reducing catheter‑time, for example, indicates a fairly strong benefit.
Remember, a statistically significant p‑value doesn’t automatically translate into a meaningful clinical benefit. Compare the absolute difference, not just the p‑value.
Landmark Bethanechol Trials You Should Know
The following table captures the most cited studies from the last decade, highlighting design, patient population, primary outcome, and headline results.
| Trial | Phase & Design | Population | Primary Endpoint | Result |
|---|---|---|---|---|
| Smith et al., 2016 | Phase II, double‑blind, placebo‑controlled | 120 postoperative urinary retention patients | Time to first spontaneous void | Mean reduction 1.9h (p=0.02); AE rate 8% |
| Lee et al., 2018 | Phase III, multicenter, 1:1 randomization | 300 patients with ileus after colorectal surgery | Time to first bowel movement | 3.4day vs 4.7day (p<0.001); nausea 12% |
| Kumar et al., 2020 | Phase II, crossover | 45 chronic urinary retention patients | Post‑void residual volume | Reduction of 45mL (p=0.04); dizziness 5% |
| Garcia et al., 2022 | Phase III, adaptive design | 210 elderly patients with neurogenic bladder | Successful voiding rate at 6h | 78% vs 61% (p=0.01); cardiac events 2% |
Notice the pattern: most studies focus on time‑to‑event outcomes and report a modest but consistent advantage over placebo. Adverse‑event rates hover between 5‑12%, with most events being mild and reversible.
How to Interpret the Data: Practical Tips
Here’s a quick checklist you can run through when evaluating any Bethanechol paper:
- Check the randomization method. True randomization reduces selection bias.
- Verify the blinding. Double‑blind designs minimize performance bias, especially for subjective outcomes like patient‑reported comfort.
- Look at the sample size calculation. Under‑powered studies can produce false‑negative results.
- Assess the clinical relevance. A 30‑minute reduction in catheter time may be statistically significant but might not change practice.
- Scrutinize the adverse‑event profile. Compare the incidence of serious events with the control arm, not just the overall rate.
- Read the subgroup analyses. Some trials show larger benefits in younger patients or those with shorter surgical times.
If a study skips any of these steps, treat its conclusions with caution.

Common Pitfalls and Misinterpretations
Even seasoned clinicians can misread Bethanechol data. Avoid these traps:
- Equating p‑value with importance: A p‑value of 0.04 on a tiny effect size doesn’t justify changing protocols.
- Ignoring drop‑out rates: High attrition (e.g., >20%) can bias results, especially if drop‑outs are linked to side effects.
- Overgeneralizing from specific patient groups: A trial limited to elective orthopedic surgery patients may not apply to emergency trauma cases.
- Neglecting the placebo effect: In bladder studies, the act of catheter removal itself can improve outcomes, inflating the apparent drug benefit.
By keeping these red flags front‑of‑mind, you’ll separate genuine signals from noise.
Next Steps: Applying the Evidence in Practice
If you’re a clinician, consider the following workflow after reviewing the literature:
- Identify the patient subgroup that matches the trial population (e.g., postoperative urinary retention after prostate surgery).
- Check contraindications (e.g., asthma, severe coronary disease) that were excluded from trials.
- Choose a dosing regimen that mirrors the successful studies (typically 10-30mg oral q8h).
- Monitor for the highlighted adverse events during the first 24hours of therapy.
- Document outcomes using the same metrics (time to first void, post‑void residual) to contribute to real‑world evidence.
Researchers can build on existing work by designing head‑to‑head trials that compare Bethanechol with newer agents like neostigmine or novel pro‑kinetic drugs.
Frequently Asked Questions
What conditions is Bethanechol approved for?
Bethanechol is FDA‑approved for postoperative urinary retention and for stimulating gastrointestinal motility in cases of postoperative ileus.
How long does it take for Bethanechol to work?
Oral doses usually begin to show effect within 30-60minutes, with peak activity around 2hours after ingestion.
What are the most common side effects?
Mild sweating, abdominal cramping, flushing, and transient hypotension are the most frequently reported adverse events.
Is Bethanechol safe for elderly patients?
Trials in patients over 70 show similar efficacy but a slightly higher rate of cardiovascular side effects; starting at the low end of the dose range is advised.
Can Bethanechol be combined with other pro‑kinetic agents?
Combination studies are limited, but the additive risk of cholinergic side effects means clinicians should be cautious and monitor cardiac function closely.
Carl Boel
October 15, 2025 AT 14:25From a pharmacoeconomic and national health‑security standpoint, the strategic deployment of Bethanechol must be anchored in rigorous, evidence‑based criteria that safeguard our healthcare sovereignty.
Critically, the drug’s muscarinic agonism at M3 receptors confers a mechanistic advantage that aligns with our nation’s emphasis on reducing catheter‑associated complications, thereby conserving valuable hospital resources.
When scrutinizing phase‑III data, any p‑value that fails to achieve a clinically meaningful reduction in catheter‑time-specifically beyond a two‑hour threshold-should be dismissed as statistically hollow.
The safety profile, while generally predictable, demands a zero‑tolerance policy for adverse events that could compromise cardiovascular stability in our aging veteran population.
Consequently, any trial reporting even a marginal rise in hypotensive episodes must be subject to heightened regulatory scrutiny before integration into standard protocols.
Shuvam Roy
October 17, 2025 AT 21:58Appreciate the detailed analysis; it’s essential we keep patient safety at the forefront while also considering the broader impacts on healthcare efficiency.
When interpreting the significance of p‑values, remember that clinical relevance often hinges on absolute risk reduction rather than mere statistical significance.
Ensuring that dosing regimens mirror those proven effective in controlled trials will help maintain therapeutic consistency across diverse patient cohorts.
Finally, continuous monitoring for cholinergic side effects-especially within the first 24 hours-remains a cornerstone of prudent clinical practice.
Jane Grimm
October 20, 2025 AT 05:31While the exposition is commendably thorough, the tone borders on sanctimonious, as if prescribing a moral code for trial interpretation.
One must question whether the emphasis on “national health‑security” eclipses the nuanced patient‑centered outcomes that truly matter.
Nevertheless, the insistence on a two‑hour catheter‑time reduction as a decisive benchmark is a nuanced, albeit idealistic, criterion.
In practice, clinicians must weigh such thresholds against real‑world variables, including comorbidities and resource constraints.
Nora Russell
October 22, 2025 AT 13:05The preceding discourse, though earnest, overlooks the stratified heterogeneity inherent in postoperative populations.
Methodologically, the cited phase‑III trials suffer from selection bias, limiting the external validity of their findings.
Moreover, the reliance on mean reductions without presenting median or interquartile ranges obscures the distribution of therapeutic benefit.
A more rigorous meta‑analytic approach, incorporating forest plots and heterogeneity statistics (I²), would substantially elevate the analytical robustness.
Meghan Cardwell
October 24, 2025 AT 20:38Indeed, the granular examination of Bethanelov’s trial architecture unveils several pivotal considerations that merit elaborate discussion.
First, the pharmacokinetic stewardship observed in phase‑I cohorts underscores a dose‑dependent absorption curve, which directly informs the titration schema employed in subsequent phases.
Second, the primary efficacy endpoint-time to first spontaneous void-exhibits a mean differential of 1.9 hours, yet this figure must be contextualized within the confidence interval bandwidth, which, as reported, spans 1.3 to 2.5 hours, indicating respectable precision.
Third, the safety dataset reveals an adverse event incidence of 8 %, predominantly mild cholinergic manifestations such as diaphoresis and abdominal cramping, thereby affirming a favorable risk‑benefit equilibrium for the intended demographic.
Fourth, the number needed to treat (NNT) of four, derived from the proportion of patients achieving voiding within six hours, conveys a clinically impactful benefit, surpassing conventional thresholds for therapeutic adoption.
Fifth, trial heterogeneity was mitigated through stratified randomization, ensuring balanced allocation across surgical subtypes, which bolsters internal validity.
Sixth, the intention‑to‑treat (ITT) analytical framework preserved the integrity of the efficacy assessment, precluding attrition bias that could otherwise inflate effect sizes.
Seventh, the comparative arm utilizing placebo illuminated a notable placebo effect, reinforcing the necessity of rigorous blinding protocols to delineate true pharmacologic impact.
Eighth, subgroup analyses disclosed a modest attenuation of efficacy in patients over 70, signaling the need for cautious dose initiation in geriatric cohorts.
Ninth, the absence of head‑to‑head comparisons with neostigmine underscores a lingering evidence gap that future investigations should prioritize.
Tenth, real‑world implementation would benefit from post‑marketing surveillance databases to track rare, idiosyncratic adverse events beyond the controlled trial milieu.
Eleventh, cost‑effectiveness modeling, integrating the reduction in catheter‑related complications, suggests a net economic gain for health systems adopting Bethanechol under the outlined parameters.
Twelfth, integration of patient‑reported outcome measures (PROMs) could further elucidate quality‑of‑life enhancements, an aspect currently underrepresented in the literature.
Thirteenth, the pharmacodynamic profile indicates a peak effect at approximately two hours post‑administration, aligning with the observed temporal window for clinical benefit.
Fourteenth, clinicians should remain vigilant for hypotensive episodes, particularly in patients with underlying cardiovascular instability, and adjust dosing accordingly.
Fifteenth, the cumulative evidence positions Bethanechol as a viable adjunct in postoperative bladder management, provided that prescribers adhere to evidence‑based dosing, monitoring, and patient selection criteria.
stephen henson
October 27, 2025 AT 03:11Great rundown! 👍
For anyone new to this, just remember: start low, go slow, and keep an eye on those vitals.
If the patient feels shaky or starts sweating profusely, dial it back.
And hey, share your outcomes – the more data we have, the better we can fine‑tune the protocol.
Namrata Thakur
October 29, 2025 AT 10:45Thanks for the coaching tip!
In simple terms, Bethanechol can be a game‑changer for early recovery, but only if we respect its power.
Imagine a patient finally able to get up and go after surgery – that’s the hopeful vision.
Let’s stay optimistic, monitor closely, and celebrate each successful void as a small victory.
Chloe Ingham
October 31, 2025 AT 18:18They’re hiding side effects on purpose.
Mildred Farfán
November 3, 2025 AT 01:51Oh sure, because the pharma lobby definitely wants us to miss out on those “hidden” side effects – thanks for the heads‑up, conspiracy theorist. 🙄