Type 1 Diabetes: Managing Autoimmune Destruction of the Pancreas
 Jan, 17 2026
Jan, 17 2026
When your body turns on its own pancreas, life changes overnight. Type 1 diabetes isn’t just about high blood sugar-it’s an autoimmune war inside your pancreas, where your immune system attacks and destroys the insulin-making beta cells. By the time symptoms appear, most people have already lost 80-90% of these cells. This isn’t a lifestyle issue. It’s not caused by eating too much sugar. It’s a misunderstood disease: type 1 diabetes is an autoimmune pancreatic disease.
What Happens Inside the Pancreas?
Your pancreas has two jobs: making digestive enzymes (exocrine function) and making insulin (endocrine function). In type 1 diabetes, only the endocrine part is targeted. Immune cells-mostly T-cells-march into the islets of Langerhans and kill the beta cells that produce insulin. This isn’t random. It’s precise. The immune system mistakes insulin, GAD65, IA-2, and ZnT8 as foreign invaders. These are proteins made by your own beta cells, but your immune system sees them as threats. This attack doesn’t happen overnight. It starts years before diagnosis. Three stages define the progression:- Stage 1: You have two or more autoantibodies, but blood sugar is normal. You don’t feel sick.
- Stage 2: Blood sugar starts to rise, but you still have no symptoms. Your body is struggling to make enough insulin.
- Stage 3: Symptoms appear-extreme thirst, weight loss, fatigue. You’re now insulin-dependent.
Why It’s Not Just ‘Juvenile Diabetes’
For decades, people called it juvenile diabetes. That name stuck because it often shows up in kids. But 50% of new cases now happen in adults. And many adults are misdiagnosed as having type 2 diabetes. That’s dangerous. Type 2 means your body resists insulin. Type 1 means you have almost none. If you’re given metformin or told to lose weight when you’re actually type 1, your blood sugar won’t improve-and your beta cells keep dying. Studies show 12% of adults with type 1 are initially misdiagnosed. Eight percent end up in diabetic ketoacidosis (DKA) because they weren’t started on insulin fast enough. Adult-onset type 1, sometimes called LADA (Latent Autoimmune Diabetes in Adults), moves slower. People might stay off insulin for a year or two. But eventually, they’ll need it. C-peptide levels tell the story: below 0.2 nmol/L at diagnosis? You’re making almost no insulin. Above 0.6? That’s more likely type 2.Genes, Triggers, and the Autoimmune Chain
You don’t get type 1 diabetes because you ate too many cookies. You get it because your genes and environment collided. The biggest genetic risk? HLA-DR3/DR4. People with this combination are 20 to 30 times more likely to develop type 1. But not everyone with these genes gets sick. Something else has to trigger it. Enter viruses. Coxsackievirus B shows up in the blood of people months before diagnosis. It’s not the virus itself that kills beta cells-it’s the immune response. The virus mimics a beta-cell protein. Your immune system fights the virus… and accidentally starts attacking your pancreas. Other triggers? Gut health. Research shows 67% of people with type 1 have less of a good gut bacteria called Faecalibacterium prausnitzii. This bug makes butyrate, a short-chain fatty acid that calms inflammation. Less butyrate? More immune chaos.
Managing Type 1 Diabetes Today
There’s no cure yet. But management has changed dramatically in the last five years. Insulin is still the foundation. Most people use either multiple daily injections (MDI) or an insulin pump. The standard is 0.5 units per kilogram of body weight per day, split 50/50 between basal (background) and bolus (mealtime) insulin. Insulin types matter. Long-acting insulins like glargine U-300 last 36 hours with less peak. Rapid-acting ones like aspart kick in within 10 minutes. These aren’t just upgrades-they’re safety tools. They reduce low blood sugar events by 40-50% compared to older human insulins. Continuous glucose monitors (CGMs) are game-changers. Devices like the Dexcom G7 (approved in 2022) give you real-time numbers, trends, and alerts. The DIAMOND trial showed users dropped their A1c by 0.4-0.6% and had far fewer hypoglycemic episodes. For parents of kids with type 1, the ability to see their child’s glucose on their phone while they’re at school? Life-saving. Artificial pancreas systems (like Tandem’s Control-IQ) combine CGMs with pumps and algorithms. They adjust insulin automatically. In a 2022 JAMA study, users spent 71-74% of the day in target range (70-180 mg/dL). People on MDI? Only 51-55%. That’s not just convenience-it’s protection against long-term damage to kidneys, eyes, and nerves.The New Frontier: Stopping the Autoimmune Attack
The biggest breakthrough since insulin? Teplizumab (Tzield). Approved by the FDA in November 2022, it’s the first drug that can delay type 1 diabetes diagnosis. It doesn’t cure. It buys time. In the PROTECT trial, people with Stage 2 type 1 (autoantibodies + rising blood sugar) who got teplizumab stayed in Stage 2 for an average of 29.8 months longer than those who didn’t. That’s over two years where they didn’t need insulin. Teplizumab works by blocking the T-cells that kill beta cells. It’s given as a 14-day IV infusion. It’s not for everyone-it’s only for Stage 2, and it’s expensive. But it’s proof that we can slow the disease. Other drugs are in trials. Abatacept (a drug used for rheumatoid arthritis) reduced C-peptide decline by 59% in recent-onset type 1. Verapamil, a blood pressure pill, preserved 30% more insulin production in a 2022 trial. And Vertex Pharmaceuticals’ stem cell therapy (VX-880) restored insulin independence in 89% of participants after 90 days. These aren’t science fiction. They’re real. And they’re coming fast.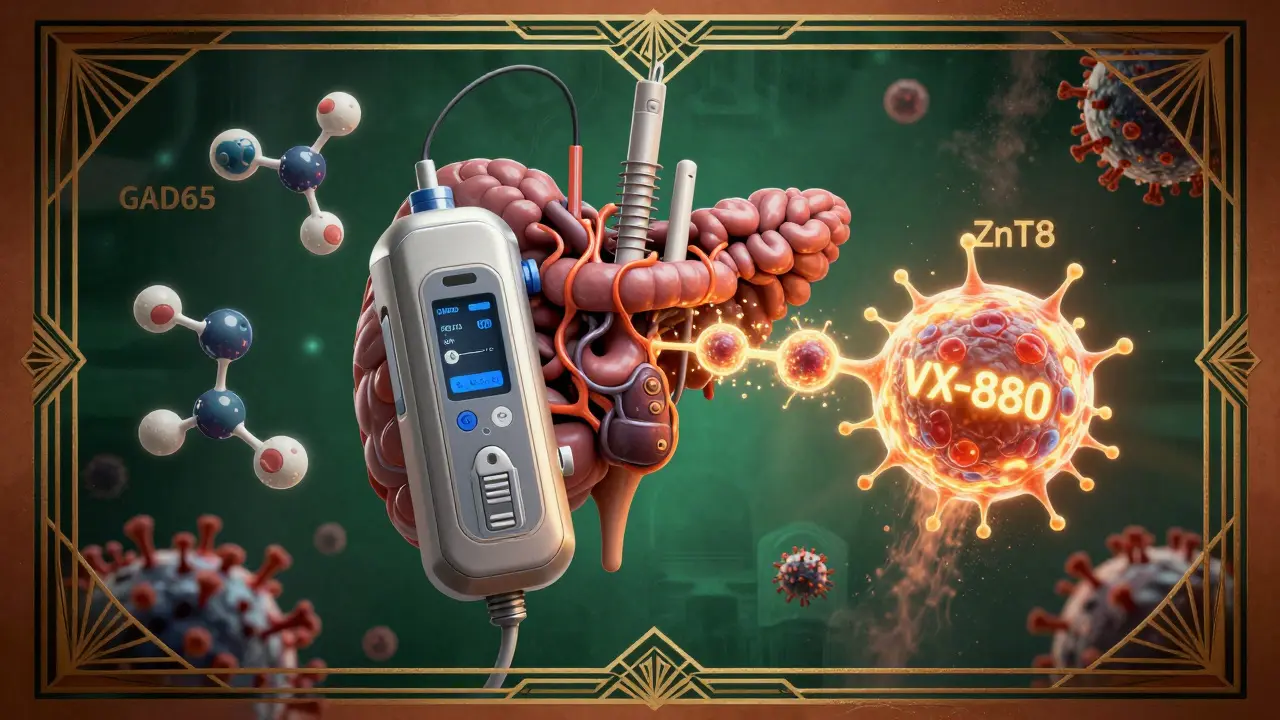
The Rare Link: Autoimmune Pancreatitis and Type 1
Most people with type 1 have no issues with their exocrine pancreas-the part that makes digestive enzymes. But in about 1 in 300 cases, something unusual happens: autoimmune pancreatitis (AIP) shows up too. AIP is when the immune system attacks the entire pancreas, not just the islets. It’s linked to IgG4 antibodies and can cause jaundice, weight loss, and abdominal pain. It’s treated with steroids-and steroids raise blood sugar. So if you have both type 1 and AIP, your insulin needs can spike overnight. This overlap is rare, but it’s important. If a person with type 1 suddenly develops unexplained belly pain, nausea, or jaundice, their doctor should check for AIP. It’s treatable. And if caught early, it can be reversed.What’s Next?
The future of type 1 diabetes isn’t just better insulin. It’s combination therapy: immunotherapy to stop the attack, plus drugs to protect or restore beta cells. The 2024 ADA/EASD guidelines call this the next frontier. We’re moving from managing symptoms to changing the disease course. The goal? Keep people in Stage 2 for decades. Help them keep their own insulin production. Eventually, restore it. For now, the best thing you can do is:- Test for autoantibodies if you have a family history.
- Use CGMs-even if you’re on injections.
- Don’t delay insulin if you’re diagnosed.
- Ask about clinical trials if you’re newly diagnosed.
Is type 1 diabetes the same as autoimmune pancreatitis?
No. Type 1 diabetes attacks only the insulin-producing beta cells in the pancreas. Autoimmune pancreatitis (AIP) attacks the exocrine part of the pancreas that makes digestive enzymes. They’re separate diseases-but in rare cases (about 0.3%), both can happen together. When they do, treatment requires both insulin and steroids, and insulin doses often need adjustment because steroids raise blood sugar.
Can type 1 diabetes be reversed?
Not yet. Once beta cells are destroyed, they don’t regenerate in adults. But new treatments like teplizumab can delay diagnosis by years. Stem cell therapies (like Vertex’s VX-880) have restored insulin production in early trials. The goal isn’t just to manage blood sugar anymore-it’s to stop the immune attack and preserve or replace beta cells.
Why do some people with type 1 diabetes still make some insulin?
Even after diagnosis, many people retain a small number of beta cells-sometimes for years. This is called residual beta-cell function. It’s measured by C-peptide levels. People with higher C-peptide have fewer blood sugar swings and lower risk of complications. New drugs like verapamil and abatacept aim to protect these remaining cells. The more you preserve, the easier management becomes.
Is type 1 diabetes hereditary?
Genes play a role, but it’s not simple inheritance. If your parent has type 1, your risk is about 5%. If your sibling has it, your risk is 7-10%. The strongest genetic link is the HLA-DR3/DR4 gene combo, which increases risk 20-30 times. But most people with these genes never develop type 1. Something else-like a virus or gut imbalance-must trigger it.
What’s the best way to monitor type 1 diabetes daily?
Continuous glucose monitoring (CGM) is now the gold standard. Devices like Dexcom G7 or Freestyle Libre give real-time glucose readings, trend arrows, and alerts for highs and lows. Studies show CGM users reduce A1c by 0.4-0.6% and cut hypoglycemia by nearly half. Even if you use insulin pens, a CGM gives you the data to make smarter decisions-no fingersticks needed.
Can diet or fasting cure type 1 diabetes?
No. Diet can help manage blood sugar, but it cannot reverse the autoimmune destruction of beta cells. Fasting, keto diets, or eliminating sugar won’t bring back insulin production. In fact, restrictive diets can increase the risk of dangerous lows. The only proven treatment is insulin, combined with modern tools like CGMs and insulin pumps. Focus on balanced nutrition-not miracle cures.
How does stress affect type 1 diabetes?
Stress raises cortisol and adrenaline, which push blood sugar up-even if you haven’t eaten. This is true for everyone, but people with type 1 can’t naturally counteract it with insulin. That’s why anxiety, illness, or sleep loss often causes unexpected highs. Tracking stress alongside glucose helps identify patterns. Many CGMs now let you log stress, sleep, and activity to see how they connect.
What’s the difference between type 1 and type 2 diabetes?
Type 1 is autoimmune: your body destroys insulin-producing cells. You make little to no insulin. Type 2 is metabolic: your body resists insulin and eventually can’t make enough. Type 1 needs insulin from day one. Type 2 may start with diet, pills, or GLP-1 agonists. C-peptide levels confirm the difference: below 0.2 nmol/L for type 1, above 0.6 for type 2. Misdiagnosing one as the other can lead to serious complications.
Jodi Harding
January 18, 2026 AT 17:30Finally, someone gets it. Type 1 isn’t a choice. It’s not laziness. It’s not sugar. It’s a biological betrayal.
Pat Dean
January 20, 2026 AT 09:04Oh here we go again with the ‘it’s not your fault’ sermon. People with type 1 are just as responsible for their health as anyone else. Stop coddling them. If they’d just eat less carbs, they wouldn’t need all this fancy tech.
Selina Warren
January 20, 2026 AT 23:12Y’all need to hear this: this isn’t just science-it’s survival. Every time someone says ‘just take insulin’ like it’s a pill for a headache, they’re erasing our reality. We’re not patients. We’re warriors with pumps, CGMs, and midnight glucose checks. And yeah-we’re tired. But we’re still here. And we’re fighting. And we’re winning.
christian Espinola
January 21, 2026 AT 05:58Teplizumab? FDA-approved? Interesting. But let’s not ignore the fact that the entire autoimmune diabetes narrative was pushed by Big Pharma to sell more insulin pumps and CGMs. The real cause? GMOs, glyphosate, and vaccines. The science is buried. Look at the funding sources. Always look at the funding.
Jake Moore
January 22, 2026 AT 16:27For anyone new to this: if you’re diagnosed, get a CGM ASAP-even if you’re on injections. It’s not optional anymore. I went from 10 fingersticks a day to zero. My A1c dropped from 8.2 to 6.1 in 4 months. The data doesn’t lie. And yes, it’s expensive-but insurance usually covers it if you ask properly.
Naomi Keyes
January 22, 2026 AT 21:15Regarding residual beta-cell function: C-peptide levels below 0.2 nmol/L are indicative of near-total beta-cell destruction, whereas levels above 0.6 nmol/L are strongly suggestive of type 2 diabetes-however, this threshold is not universally standardized across all laboratories, and some assays may vary by ±0.1 nmol/L depending on calibration methods and assay type (e.g., RIA vs. ELISA). Additionally, the presence of exogenous insulin can interfere with C-peptide interpretation, necessitating careful clinical correlation.
kenneth pillet
January 23, 2026 AT 05:16My kid was diagnosed at 7. We didn’t know any of this. Got lucky with a good endo. CGM saved us. No drama. Just facts. Thanks for the post.
Max Sinclair
January 23, 2026 AT 19:01Love how this post avoids the usual ‘it’s a lifestyle disease’ nonsense. Seriously, why do people still think this? I’ve seen adults misdiagnosed as type 2 for years. One guy went into DKA because he was told to ‘lose weight and cut sugar.’ Dude had zero insulin production. That’s not negligence-it’s ignorance.
Chuck Dickson
January 23, 2026 AT 23:02To everyone reading this: you’re not alone. I’ve been doing this for 27 years. I’ve had every complication you can imagine-nerves, kidneys, eyes. But I’m still here. Why? Because I learned to listen to my body. Because I use my CGM like a compass. Because I found my people. You don’t have to be perfect. You just have to keep showing up.
Jay Clarke
January 24, 2026 AT 11:39Let’s be real-this whole ‘autoimmune war’ metaphor is just poetic nonsense. It’s not a war. It’s a glitch. Your body’s software has a bug. The immune system didn’t ‘turn on’-it got corrupted. And yeah, teplizumab is cool, but it’s just a patch. We need a full OS update. Not more insulin. Not more pumps. We need a reset button.
Ryan Otto
January 25, 2026 AT 20:18Interesting how this ignores the global disparity. In South Africa, most people with type 1 die within two years of diagnosis because insulin is unaffordable. This post reads like a luxury ad for rich Americans with Dexcom G7s. The real crisis isn’t in the pancreas-it’s in the economic system.
rachel bellet
January 26, 2026 AT 21:07Let’s not romanticize this. Type 1 diabetes is a chronic, debilitating, life-altering condition that reduces life expectancy by 8–10 years on average. The ‘resilience’ narrative is toxic. It places the burden of survival entirely on the individual while ignoring systemic failures in healthcare access, insurance coverage, and pharmaceutical pricing. You’re not a warrior-you’re a victim of broken systems.
Robert Davis
January 28, 2026 AT 16:17Just read the part about AIP. My uncle had that. He got steroids, his blood sugar went nuts, and they didn’t connect it to his type 1. Took him six months to get the right diagnosis. He almost lost his pancreas. This needs to be shouted from rooftops. Doctors don’t know this stuff.